 What is a
Crystal?
What is a
Crystal?
What is a crystal made out of and what holds the pieces together ?
Crystals are built out of atoms. The fundamental building blocks for atoms are protons, neutrons and electronsAtoms are listed and arranged in the periodic table according to their number of protons (increasing left to right across the rows).
You should recognize the names and symbols for elements that make up, and give color to, the important gems.
In general, the elements found in gemstones are those that are relatively abundant in the crust (however, this is not always true!).
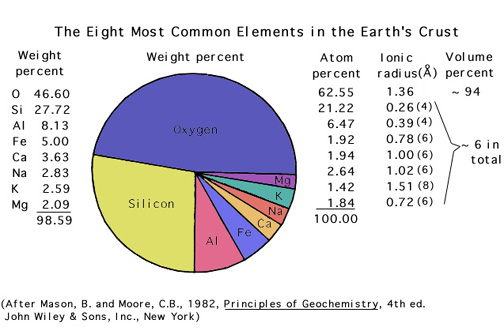
Please study this list:
What are ions and how do they form?
Ions form when atoms lose (cations) or gain (anions) electrons.Cations are positively charged ions
Anions (negatively charged ions) charged ions.
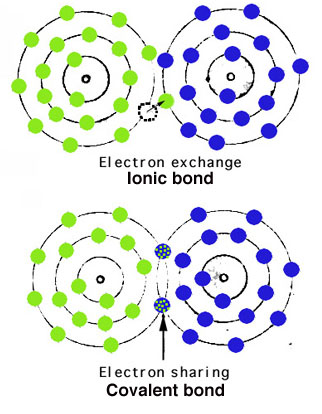
Atoms are held together in crystals by atomic bonding.
The most important types of bonds are via electron exchange (ionic) or electron sharing (covalent), as shown simplistically to the right.
How are minerals grouped?
Minerals are grouped firstly based on what they contain.These are the most important groups for this course:
- silicates - silica (Si)-rich minerals
- Examples include amethyst (quartz), tourmaline, beryl
- oxides - anion is oxygen (O)
- Examples include corundum (ruby and sapphire) and rutile
- carbonates - contain carbon and oxygen (C)
- Examples include calcite and aragontite (in pearls)
- phosphates - contain phosphorus (P)
- borates - contain boron (B)
- sulfides and sulfates - contain sulfur (S)
- halides - contain chlorine (Cl) or other elements from group VIIa in the periodic table
What is a crystal?
Something is crystalline if the atoms or ions that compose it are arranged in a regular way (i.e, a crystal has internal order due to the periodic arrangement of atoms in three dimensions). Gems are described as amorphous if they are non-crystalline.Crystals characterized by well developed crystal faces (external surfaces) are described as euhedral . Crystals do not always show well developed crystal faces seen on euhedral examples.
A crystal is built up by arranging atoms and groups of atoms in regular patterns, for example at the corners of a cube or rectangular prism.
The basic arrangement of atoms that describes the crystal structure is identified. This is termed the unit cell.
Crystals must be charge balanced. This means that the amount of negative charge must be compensated by the same amount of positive charge.
Example:
Al 3+ and O 2-: These are combined as Al2O3 (two aluminums per three oxygens). Make sure you understand why it is not AlO (one aluminum per one oxygen!)

How are cations and anions arranged?
The atomic cluster consisting of a regular arrangement of anions around a central cation (or visa versa) is described as a coordination polyhedra. Coordination polyhedra are larger building blocks than individual atoms.Coordination arrangements are determined by relative sizes of cations (+) and anions (-).
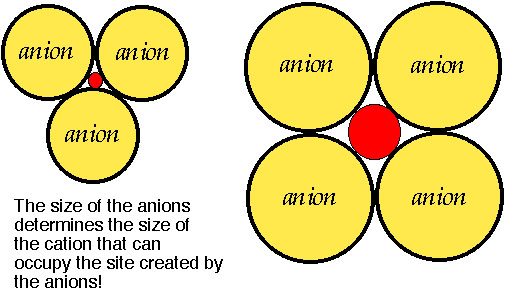
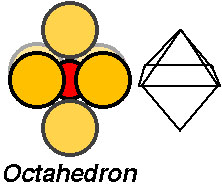
How are coordination polyhedra arranged ?
Crystals are formed by linking of coordination polyhedra:Imagine building a crystal by linking the building blocks (tetrahedra or octahedra) into chains or frameworks.
Example: silicate polyhedra may be linked by sharing of oxygen atoms (corners) between two adjacent silicon tetrahedra.
Not required, but for your information: other polyhedra (e.g., octahedra)
may be linked by sharing edges (e.g., two adjacent oxygens are shared between
two octahedra) and faces (e.g., three adjacent oxygens are shared between
two adjacent octahedra).
Is more than one arrangement of coordination polyhedra possible?
YES!At different temperatures and pressures, different arrangements of the same elements are stabilized.
The term "polymorphs " refers to different crystal structures with the same elemental composition (elements are arranged differently).
Symmetry and crystals
The symmetry involves the pattern of arrangement of atoms."Symmetry" refers to sameness. Here are some examples of symmetric patterns of objects to illustrate symmetry!
All minerals are assigned to one of six (if we group rhombohedral and
hexagonal together) crystal systems. Crystal systems are determined
based on the symmetry of the mineral.
| CRYSTAL SYSTEM |
| Cubic |
| Hexagonal |
| Tetragonal |
| Orthorhombic |
| Monoclinic |
| Triclinic |
 Note that the crystal's external form is the
direct result of addition of growth
by addition of groups of atoms (unit cells) in a fixed arrangement!
Note that the crystal's external form is the
direct result of addition of growth
by addition of groups of atoms (unit cells) in a fixed arrangement!
Examples of crystals:- silicate and oxide minerals
Silicate minerals: Silicates have structures containing abundant silica tetrahedra, i.e., a tetrahedron with a Si at the center, surrounded by four oxygen anions.There are thousands of naturally occurring compounds (minerals). Most are not suitable for use as gems (see What is a Gem).Many silicates contain linkages of silica tetrahedra. This forms the backbone of the structure. Silicate minerals are classified based upon the way in which the tetrahedra are linked together.
Gem examples of the extremes of this classification system are:
Oxide minerals: Simple oxide minerals consist of metals and oxygen. Examples include corundum (Al2O3) and hematite (Fe2O3). These contain metals (aluminum or iron) in a six coordinated site (octahedral site) formed by oxygen anions.
For a relatively complete listing of mineral names and the chemical formulae, click here