Research in Mineralogy and Materials Science:
Nanocrystals and the environment
Finely crystalline materials
The Banfield research group studies materials
with particle diameters in the few nanometer size range. Small particles
are characterized by a large surface area to volume ratio and higher total
energy due to excess energy associated with ions at the surfaces.
Why do we care about this? Nanophase materials are
extremely common products of microbial mineralization and chemical weathering
reactions. These nanocrystalline oxides and sulfides (etc.) account for
much of the reactive surface area in the Earth's near surface environment.
They are probably also the predominant component of Martian Dust.
Thus, nanocrystalline materials have the potential to dramatically impact
the chemistry and physics of planetary surfaces.
How are small crystals different from big crystals?
The surface energy of a macroscopic crystal is an insignificant
fraction of its total energy. However, this is not so for very small crystals.
In fact, the phase stability of two polymorphs may be reversed because
their surface energies are different. In the Banfield group, Amy Gribb
and Dr. Hengzhong Zhang have worked on the size
dependence of phase stability in
nanocrystalline titania (TiO2).
-
Gribb, A.A. and Banfield, J.F. (1997) Particle size
effects on transformation kinetics and phase stability in nanocrystalline
TiO2. American Mineralogist, 82, 717-729
-
Zhang, H. and Banfield, J.F. (1998) A model for exploring
particle size and temperature dependence of excess heat capacities of nanocrystalline
substances. NanoStructured Materials, 10, 184-195.
-
Zhang, H. and Banfield, J.F. (1998) Phase stability in the
nanocrystalline TiO2 system "Phase Transformations and Systems Driven Far
From Equilibrium", Eds. E. Ma, P. Bellon, M. Atzmon, R. Trivedi, MRS, 619-624
-
Zhang, H. and Banfield, J.F. (1998) Thermodynamic analysis
of phase stability in nanocrystalline titania. Journal of Materials
Chemistry, 8, 2073-2076.
Size has many other consequences for minerals and
materials reactivity. We are continuing or work to investigate how
particle
size affects phase transformation kinetics. The following
papers by Dr. Hengzhong Zhang and from the Ph.D. research of R. Lee Penn
illustrate some of the concepts we have pursued.
-
Zhang, H. and Banfield, J.F. (1999) A new kinetic model for
the anatase-to-rutile phase transformation in nanocrystalline material
revealing a second order dependence on the number of particles. American
Mineralogist, 84, 528-535.
-
Zhang H. and J. F. Banfield, Phase transformation of nanocrystalline
anatase-to-rutile via combined interface and surface nucleation (2000)
Journal
of Materials Research, 15(2), 437-448.
-
Zhang, H. and Banfield, J.F. (2000) Understanding polymorphic
phase transformation behavior during growth of nanocrystalline aggregates:
insights from TiO2. Journal of Physical Chemistry B., 104, 3481
-3487
Size has other implications. Small
crystals grow by different pathways than big crystals. The crystal
growth pathway that may predominate under some conditions has been described
as "oriented attachment" or "oriented aggregation". In this pathway,
crystals no more than a few nanometers in diameter aggregate and rotate
so that adjacent surfaces share the same crystallographic orientation.
The pair of adjacent interfaces are eliminated and the pair of nanoparticles
are converted to a larger single crystal.
-
Penn, R.L. and Banfield, J.F. (1999) Morphology development
and crystal growth in nanocrystalline aggregates under hydrothermal conditions:
insights from titania. Geochim. Cosmochim. Acta, 63, 1549-1557.
Crystal growth in nanocrystalline materials via novel
pathways has other implications. Nanocrystal aggregation-based
growth leads to formation of defects. These
include point (impurity, vacancy), line (dislocation), and planar (twins,
stacking faults) defects.
-
Penn, R.L. and Banfield, J.F.
(1998) Oriented attachment and growth, twinning, polytypism, and
formation of metastable phases: insights from nanocrystalline TiO2. American
Mineralogist, 83, 1077-1082.
-
Penn R.L. and Banfield J.F. (1999) Formation of rutile nuclei
at anatase {112} twin interfaces and the phase transformation mechanism
in nanocrystalline titania, American Mineralogist, 84, 871- 876.
-
Penn, R.L. and Banfield, J.F. (1998) Imperfect
oriented attachment: a mechanism for dislocation generation in defect-free
nanocrystals. Science, 281, 969-971.
Small crystals may have crystal chemically different sites
on their surfaces. Furthermore, for thermodynamic reasons, they nanocrystals
may have a stronger tendency to adsorb ions onto their surfaces.
-
Zhang, H., Penn, R.L., Hamers, R.J., and Banfield, J.F (1999)
Enhanced Adsorption on Surfaces of Nanocrystalline Materials. J. Phys.
Chem. B. 103, 4656-4662.
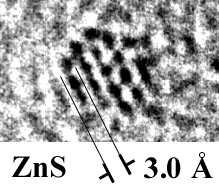
We have conducted most of our work to date on synthetic TiO2
compounds. However, recently, we have demonstrated that the concepts
developed through study of this model system apply well to phenomena that
occur in natural FeOOH and ZnS. Only a subset of this work is complete,
and most is not yet published.
We continue to explore size dependent properties and behavior
of nanocrystals, and will focus future efforts on environmentally important
compounds and processes, and on the interactions between nanocrystals and
polymers (e.g., biomineralization).
-
Banfield, J.F., Welch, S.A., Zhang, H., Ebert, T.T., and
Penn, R.L. (2000) The role of aggregation in crystal growth and transformations
in nanophase FeOOH biomineralization products. Science, 289, 751-754.
The researchers currently continuing this work include
Hengzhong Zhang Michael
Finnigan, Masha Nesterova, and Forrest Huang
Structure and microstructure analysis
Considerable effort within our group has been devoted
to identification of microstructures in minerals, and determination of
their consequences for mineral reactivity and properties.
-
Banfield, J.F. and Murakami, T. (1998) Atomic-resolution
transmission electron microscope evidence for the mechanism by which chlorite
weathers to 1:1 semi-regular chlorite-vermiculite. American Mineralogist,
83, 348-357
-
Janney, D.E. and Banfield, J.F. (1998) Cation ordering
in oxidized olivine. American Mineralogist, 83, 799-810.
-
Kogure, T. and Banfield, J.F. (1998) Direct identification
of the six polytypes of chlorite characterized by semi-random stacking.
American Mineralogist, 83, 925-930.
Check out some recent publications
from our group!
e-mail me
if you would like
more information.
Check out our work on Geomicrobiology
and Geochemistry

