 Diamonds and Diamond Simulants Diamonds and Diamond Simulants
|
Quick Links
|
Remarkable facts:
-
All diamonds are at least 990,000,000 years old.
Many are 3,200,000,000 years old (3.2 billion years)!!!
How do we know this?
Age: from Carbon dating? NO! C-dating
only works for very young carbon. You need to use other radioactive decay
schemes (e.g., uranium-lead) to date inclusions in diamonds. Inclusions
used for dating are around 100 microns in diameter (0.1 mm).
-
Diamonds are formed deep within the Earth: between 100 km and 200 km
below the surface.
Diamonds form under remarkable conditions!
-
The temperatures are about 900 - 1300 C in the part of the Earth's mantle
where diamonds form.
-
The pressure is between 45 - 60 kilobars. (kB)
-
50 kB = 150 km = 90 miles below the surface
-
60 kB = 200 km = 120 miles below the surface
-
Diamonds are carried to the surface by volcanic eruptions.
The volcanic magma conduit is known as a kimberlite pipe or diamond
pipe. We find diamonds as inclusions in the (rather ordinary looking) volcanic
rock known as kimberlite.
NOTE: The kimberlite
magmas that carry diamonds to the surface are often much younger than the
diamonds they transport (the kimberlite magma simply acts as a conveyer
belt!).
-
Diamond is made of carbon (C), yet the stable form (polymorph) of carbon
at the Earth's surface is graphite.
-
To ensure they are not converted to graphite, diamonds must be transported
extremely rapidly to the Earth's surface.
It is probable that kimberlite lavas carrying diamonds
erupt at between 10 and 30 km/hour (Eggler, 1989). Within the last few
kilometers, the eruption velocity probably increases to several hundred
km/hr.
-
Diamond is the hardest material.
Diamond is the hardest gem on the MOHS
harness scale and graphite (also made from carbon atoms) is the softest!
Given that both diamond and graphite are made of carbon, this may seem
surprising.
The explanation is found in the fact that in diamond the carbon atoms
are linked
together into a three-dimensional network whereas in graphite, the
carbon atoms are linked
into sheets with very little to hold the sheets together (thus the
sheets slide past each other easily, making a very soft material).
-
Diamonds are found in many localities,
both overseas and in the US.
|
Rarity
Basic Data
Famous Diamonds
Color
Clarity
Cut
Carat Weight
Treatment
Simulants
Synthetics
Famous diamonds
This is just for fun -- not required information!
|
How rare are diamonds?
How many grams do you need to mine to get 5 grams of diamonds?
(5g/1000 kg) @ 1000 g/kg = 5 g /1,000,000 g!
But only 20 % are gem quality (80 % of these are sold in a "managed
selling environment") and the remainder are used for industrial purposes
(this material is known as "bort" or "carbonado" (carbonado is finer)).
|
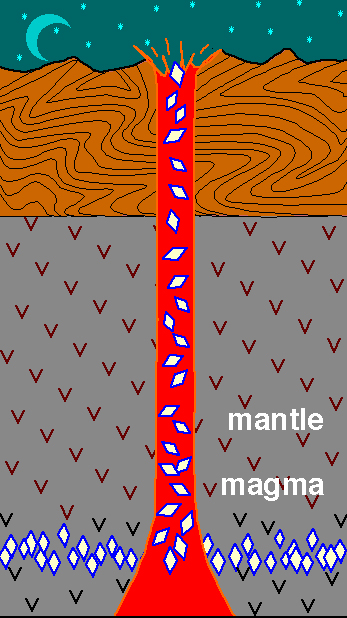
This movie
(68 k) emphasises that diamonds do not form in the kimberlite magma,
but are carried up to the surface by the magma.
|
Basic Data
Hardness = 10
Crystal System = cubic
This is what crystals look like before they are faceted: note their natural
octahedral
shape! Uncut diamonds are also found in cubic
forms.
Diamond has four good cleavages, thus diamonds tend to cleave on
impact.
Refractive Index = 2.42
Dispersion=0.044
Specific Gravity = 3.52
|


|
Value
The 4 "C" words are used to summarize the value determining factors:
The required basic information describing what is meant by these terms
is provided below.
-
Color
is determined by
'grading'
visual comparison with 'knowns' or by instrumental means.
-
Consider the amount of yellow color (yellowish color decreases the value
of a "colorless" stone). In order of increasing yellow content:
blueish-white -> white -> silver -> yellow
-
'Fancy', or strongly colored
stones have their own appeal and special value.
-
Colored diamonds may be
yellow, green or
brown,
green or shades of pink.
-
Larger
pink diamonds are quite rare and currently very expensive.
-
Natural blue diamonds contain the element boron (B), and this changes the
conductivity of the diamonds. Natural yellow diamonds contain the element
nitrogen (N).
|
 |
-
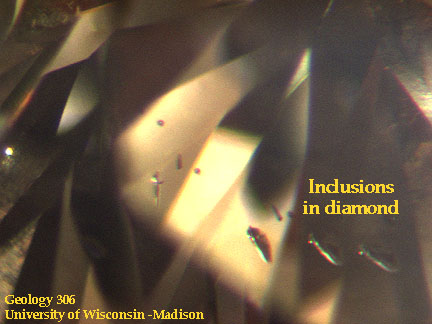
Clarity
is decreased by the presence of blemishes or flaws, scratches, nicks, 'naturals'
(the original surface of an uncut stone).
-
There are many systems of nomenclature.
-
Some terms include:
-
perfect
-
flawless
-
imperfect
-
very slightly included
-
very very slightly included
|
IF
|
VVS1
|
VVS2
|
VS1
|
VS2
|
SI1
|
SI2
|
I1
|
I2
|
I3
|
|
internally flawless
|
very, very slightly included |
|
very slightly included |
|
slightly included |
|
|
|
imperfect
|
-
other descriptions:
- "Perfect," "internally flawless," and "flawless" are not synonymous.
"Flawless" is reserved for diamonds having no visible inclusions under 10x
magnificantion and having no external blemishes of any kind.
- Clarity grades refer to what is visible at 10x magnification. With
sufficient magnification, inclusions will be revealed in any diamond.
"Perfection" is relative.
- "pique" (used below and in older literature) is an old trade term and
has been supplanted by the GIA-developed international standard, from IF
to I3.
-
"first pique" inclusions readily recognizable at 10x mag., not significantly
diminishing brilliance
-
"second pique" larger inclusions, can be seen with naked eye
-
"third pique" many large inclusions, diminishing brilliance
-
Examples of clarity-reducing inclusions:
|

|
-
Cut:
Facets are placed so as to maximize the brilliance and fire of a stone.
-
Remember that in the first lecture we talked about how the proportions
of a faceted gemstone are determined based on the refractive index?
-
Review the basic concepts:
-
Refraction
is dependent upon the wavelength.
-
Refractive Index (RI) is proportional to wavelength; red RI < violet
RI (dispersion is due to the different amounts different wavelength are
bent).
-
Fire,which
is seen as rainbows and glints of color, is due to dispersion (a consequence
of the placement of faces on the crown to take advantage of the prism
effect).
- The brilliant
cut (modern round brilliant cut or Tolkowsky cut) is a typical cut
chosen for diamonds. Tolkowsky determined the optimal proportions are
such that the table width is 53% of the diameter of the cut stone.
Appraisers will penalize diamonds with tables above 64%. Significant
deviations, up to table widths of more than 70% are not uncommon.
-
There are many alternative diamond cuts.
-
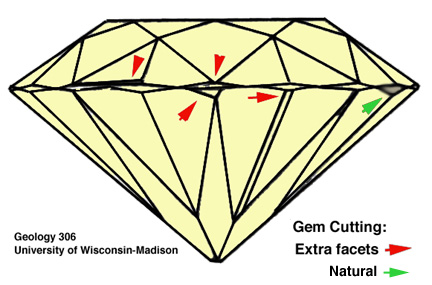
A poorly cut stone is characterized by poorly chosen proportions (poor
optimization of brilliance and fire or, worse still, leakage of light from
the pavillion). Misplaced facets, extra facets, and problems at facet
junctions are also characteristics that reduce the quality of "cut".
-
Ranking:
VERY
GOOD ... GOOD .... MEDIUM ... POOR
|
Review the light
path in a correctly cut gem!

|
-
Carat Weight
-
Recall: 1 carat = 0.2 g, thus 5 carats=1g
-
For example, compare
the size of a one point diamond to that of a 0.67 carat diamond.
Just FYI: This
site explains the GIA grading report used for diamonds, including information
on desirable
characteristics. |
|
Other issues: Treatment, simulants, synthetics
Treatments:
-
filling of cracks
Surface
cracks and cleavages reaching the surface are often filled with a
glass-like material.
Identification: when examined with an optical microscope, filled stones
will show:
-
greasy appearance
-
flash effects
-
bubbles
Problem: Filling does not always resist polishing and cleaning
-
drilling of inclusions
Drilling inclusions
involves using a laser to drillin into the inclusion. Solutions can be
poured into the resulting "hair-width" diameter hole to
bleach colored inclusions. This is comparaed to getting a filling in
your tooth.
-
irradiation
Irradiation is used to change the color of the diamond. A common color
produced by irradiation is green.
Early attempts: beginning of 20th Century: diamonds exposed to radium
- the problem was that the diamonds remained radioactive! However, modern
irradiation treatments do not produce radioctive stones.
Irradiation involves the use of devices such as:
-
linear accelerators
-
gamma ray facilities
-
nuclear reactors
Detection of irradiation treatment:
Electron irradiation only changes the surface of the stone. Thus, it
produces a concentration
of color where the gemstone is thin. For example, electron irradiation
produces a color concentration at the culet or keel line of the faceted
gem.
|
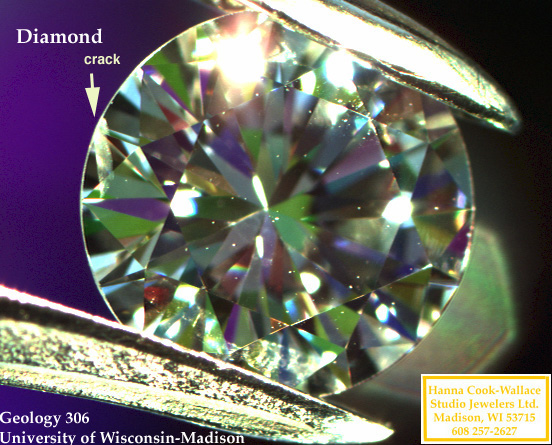
Above, a diamond with a surface crack. Below, examples of
irradiated diamonds.
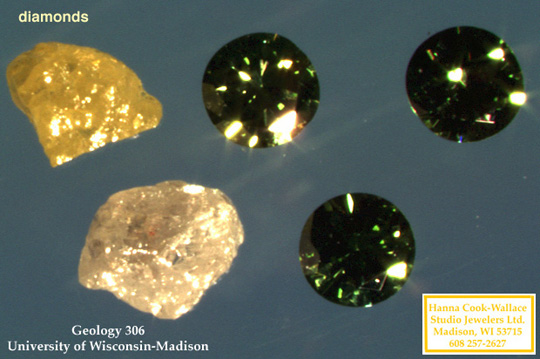
|
Simulants - simulate the appearance
of diamond
The distinction between a synthetic diamond (man-made diamond consisting
of carbon atoms arranged in the typical diamond structure) and a diamond
simulant (not a carbon compound with the diamond structure) is very
important!
In order of increasing R.I., the most common simulants are:
-
YAG = yttrium aluminum garnet
-
GGG = gadolinium gallium garnet
-
CZ
= cubic zirconia
-
Strontium titanate
-
diamond.
This mnenonic can be used to memorize the common diamond simulants in the
above order:
You go
crazy
staring
at
diamonds.
Again: Simulants (look alikes) differ from synthetics (synthesized by
humans!)!
Another diamond simulant, synthetic moissanite (Silicon carbide
or carborundum) was introduced to the jewelry market in 1998; manufactured
by C3 Inc. and Cree Research. It has become the gold standard for
diamond simulants in the last few years.
Source: Jewelers of America
| Crystal Structure |
hexagonal |
| HARDNESS |
9.5 |
| R.I. |
2.65-2.69 |
| Specific gravity |
3.17-3.20 |
Simulants
are distinguished from diamonds using measurement or observation of
various properties, such as:
-
R.I.
-
"Read through effect"
-
Dispersion
-
Hardness
-
Specific Gravity
-
Reflection pattern
-
Shadow
patterns
Note: not all diamond
simulants have been around for the same length of time! |

|
|
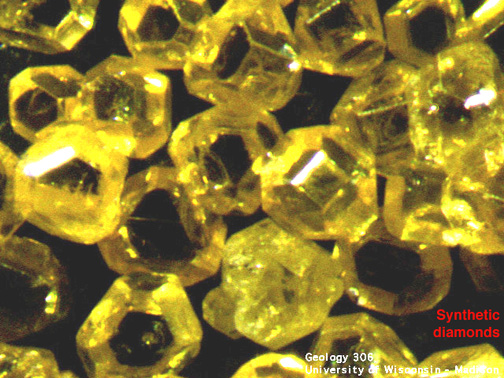
Synthetic diamonds are often yellowish
in color (rarely used for gem purposes, more commonly used as diamond grit
for industrial purposes. Modern synthesis of thin film diamond has other
industrial applications).
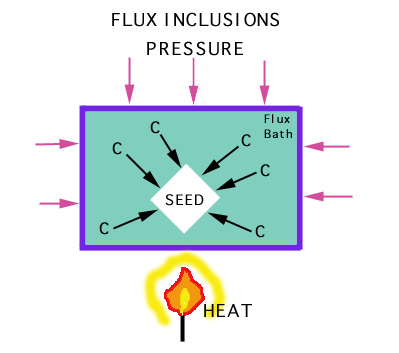
A 5 mm diamond (0.5 carat) takes over a week to grow. Synthesis requires:
Synthetic diamonds can sometimes be distinguished from natural diamonds
by the presence of flux
inclusions (Ni, Al or Fe). |
|












 Diamonds and Diamond Simulants
Diamonds and Diamond Simulants
