WHAT IS A CRYSTAL?


Crystals are built out of atoms. The fundamental building blocks for atoms are protons, neutrons and electrons
Atoms are listed and arranged in the periodic table according to their number of protons (increasing left to right across the rows).
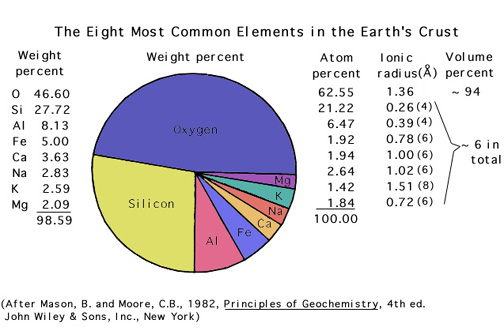
You should recognize the names and symbols for elements that make up, and give color to, the important gems: Please study this list:
In general, the elements found in gemstones are those that are relatively abundant in the crust (however, this is not always true!).
Important:- ions form when atoms loose (cations) or gain (anions) electrons. Thus:
Cations are positively charged ions
Anions (negatively charged ions) charged ions.
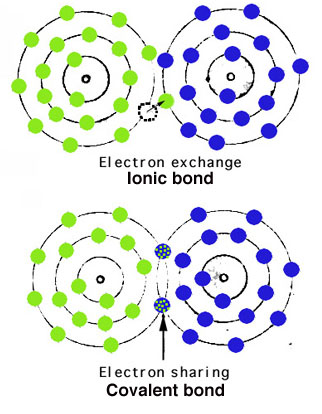
Atoms are held together in crystals by atomic bonding.
The most important types of bonds are via electron exchange (ionic) or electron sharing (covalent), as shown simplistically:
Minerals are grouped firstly based on what they contain :-
These are the most important groups for this course:
Other other groups we will not talk about here (not required for this course):
Something is crystalline if the atoms or ions that compose it are arranged in a regular way (i.e, a crystal has internal order due to the periodic arrangement of atoms in three dimensions).
Gems are described as amorphous if they at non-crystalline.

Crystals characterized by well developed cr ystal faces (external surfaces) are described as euhedral . Crystals do not always show well developed crystal faces seen on euhedral examples.
A crystal is built up by arranging atoms and groups of atoms in regular patterns, for example at the corners of a cube or rectangular prism.
The basic arrangement of atoms that describes the crystal structure is identified. This is termed the unit cell.
Crystals must be charge balanced. ALWAYS! This means that the amount of negative charge MUST be compensated by the same amount of positive charge.
Example:
The atomic cluster consisting of a regular arrangement of anions around a central cation (or visa versa) is described as a coordination polyhedra. Coordination polyhedra are larger building blocks than individual atoms.
Examples include:
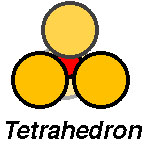
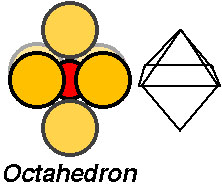
Coordination arrangements are determined by relative sizes of cations (+) and anions (-). Here is a simple explanation of why this is so!
Crystals are formed by linking of coordination polyhedra:
Imagine building a crystal by linking the building blocks (tetrahedra or octahedra) into chains or frameworks.
Example: silicate polyhedra may be linked by sharing of oxygen atoms (corners) between two adjacent silicon tetrahedra.
Not required, but for your information: other polyhedra (e.g., octahedra) may be linked by sharing edges (e.g., two adjacent oxygens are shared between two octahedra) and faces (e.g., three adjacent oxygens are shared between two adjacent octahedra).
YES! At different temperatures and pressures, different arrangements of the same elements are stabilized.
The term
"POLYMORPHS" refers to different crystal structures with the same elemental composition (elements are arranged
differently).
The symmetry involves the pattern of arrangement of atoms.
"Symmetry" refers to sameness. Here are
some examples of symmetric patterns of objects to illustrate symmetry!
All minerals are assigned to one of six (if we group rhombohedral and hexagonal together)
crystal systems. Crystal systems are determined based on the symmetry of the mineral.
The six groups, arranged in order of decreasing symmetry, are:
Is more than one arrangement of coordination polyhedra possible?
Symmetry and crystals
| CRYSTAL SYSTEM | Cubic |
| Hexagonal | Tetragonal | Orthorhombic | Monoclinic | Triclinic |
 Note that the crystal's external form is the direct result of addition of growth by addition of groups of atoms (unit cells) in a fixed arrangement!
Note that the crystal's external form is the direct result of addition of growth by addition of groups of atoms (unit cells) in a fixed arrangement!
Silicate minerals
Silicates have structures containing abundant silica tetrahedra, i.e., a tetrahedron with a Si at the center, surrounded by four oxygen anions.
Many silicates contain linkages of silica tetrahedra. This forms the backbone of the structure.
Silicate minerals are classifiedbased upon the way in which the tetrahedra are linked together.
Oxide minerals
Simple oxide minerals consist of metals and oxygen.
Examples include corundum (Al2O3) and hematite (Fe2O3).
These contain metals (aluminum or iron) in a six coordinated site (octahedral site) formed by oxygen anions.
There are thousands of naturally occurring compounds (minerals). Most are not ssuitable for use as gems (see 'What is a Gem").
For a relatively complete listing of mineral names and the chemical formulae, click here
![]() "Where Do Gems Form and Where Are They Found
?"
"Where Do Gems Form and Where Are They Found
?"
![]()
![]()
OTHER TOOLS