Mineralogical Composition of the Earth’s Crust
1. Nesosilicates
2. Inosilicates
3. Phyllosilicates
4. Tectosilicates
5. Oxides
6. Carbonates
7. Sufates
8. Halides
Silica Tetrahedron
This is a figure of quartz, but it also illustrates the repeating units of 1 Si (+4) with 4 O (-2). The unit has a net charge of –4 – but in quartz, due to sharing of O, the net charge is 0.
Aluminum Octahedron
Here the Al octahedron
is represented by the mineral gibbsite (a series of repeating Al-octas).
In the Al-octaheron, one Al (+3) is surrounded by 6 O (-2) for a net negative
charge of –3.
Nesosilicates
Nesosilicates are in some ways the simplest of the silicates, involving a basic structure of a Si tetrahedron surrounded by cations (mainly Fe+2 or Mg+2) which neutralize the net negative charge. The cations link to the tetrahedron primarily via ionic bonds, and are thus subject to easy removal by acids in soil solutions and surface waters.
The example illustrated below is
foresterite, a nesosilicate in which the balancing cation is Mg+2.
All nesosilicates form/crystallize at high temperatures and are found in
igneous rocks. Additionally, they are common components of
mafic rocks such as basalt (extrusive igneous rock).
 Foresterite
Foresterite
Inosilicates
Inosilicates are silicates with a more complex tetrahedral linkage than
nesosilicates. In these minerals, adjoining tetrahedron
share an O, thereby forming chains. These chains have a lower net
negative charge (per Si) due to a reduced O/Si ratio. The remaining net
negative charge is balanced by an array of cations.
Below are examples of two types of inosilicates: single chain types (pyroxenes) and double chains (amphiboles). Because amphiboles have an even lower O/Si ratio, they are more resistant to weathering.
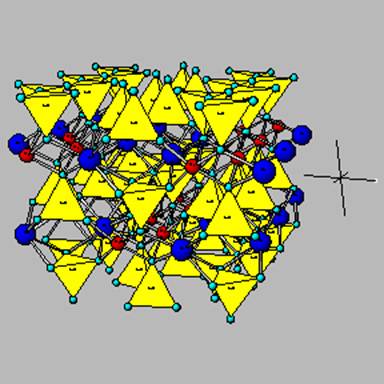 The pyroxene diopside
The pyroxene diopside
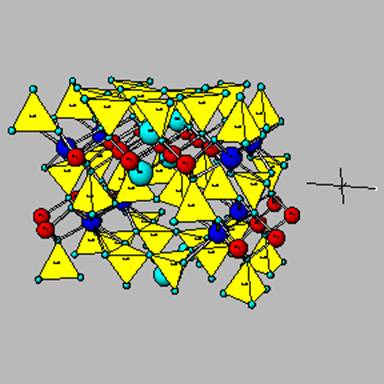 The amphibole tremolite.
The amphibole tremolite.
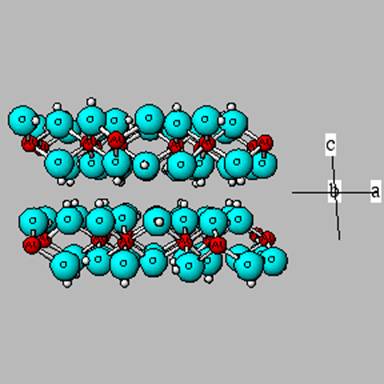
Phyllosilicates
In this mineral group, Si tetrahedrons are are linked 2 dimensionally into sheets. Additionally, between two planes of Si tetrahdrons is an octahedral layer (either filled with Al (as one end member) or with some divalent cation (Fe+2, Mg+2) at another extreme).
Phyllosilicates form at cooler temperatures than the minerals described so far. Additionally, they have a lower 0/Si ratio, thereby necessitating a need for fewer cations (and thereby making them more resistant to weathering). As a wrinkle to the weathering trends, the phllosilicates that contain Fe+2 (and any mineral that contains reduced Fe) is subject to enhanced weathering since Fe+2 is very subject to oxidation in soils – which creates a charge imbalance and the need to “eject” an Fe, which disrupts the mineral structure.
 This is muscovite. Note that
the octahedral positions are “filled” with Al+3.
This is muscovite. Note that
the octahedral positions are “filled” with Al+3.
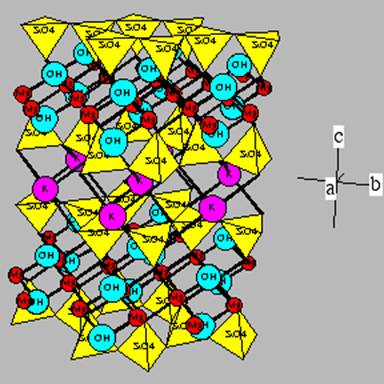 This is phlogopite. Note that
the octahedral positions are completely filled with Mg+2.
This is phlogopite. Note that
the octahedral positions are completely filled with Mg+2.
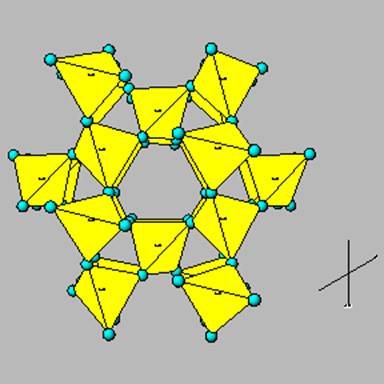
Tectosilicates
The tectosilicates represent the most complex form of Si tetrahedral linkages: a 3d sharing of O. The ideal example of this concept is quartz, a 3d linkage of O which results in a completely neutral charge, no need for counterbalancing cations, and a very strong resistance to chemical weathering.

This is quartz.
In nature, it is very common for Al (one of the earth’s major elements)
to “substitute” for Si in the tetrahedral configuration (Al has about the
same size and position on the periodic table). However, because Al has one
less positive charge than Si, the addition of Al in place of Si creates a
net negative charge that must be couterbalanced by a cation.
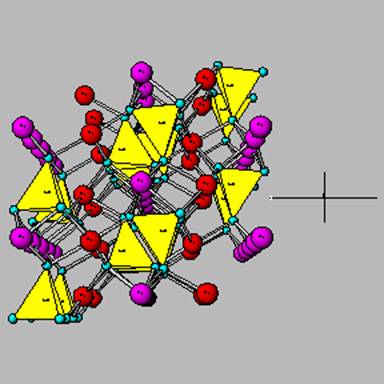
The tectosilicates that contain Al are commonly called the plagioclases (or feldspars ). The plagioclases have two end-members: one with all Na+ as a balancing cation (and hence low Al additions) or with all Ca+2 as a balancing cation (and hence more Al). Which is more susceptable to chemical weathering?
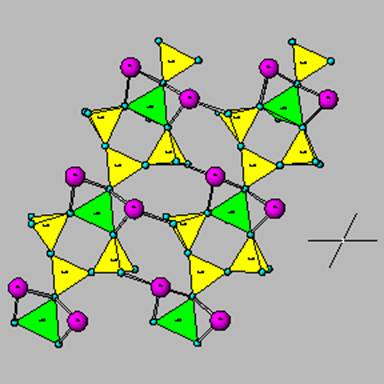 This is albite, the Na plagioclase.
This is albite, the Na plagioclase.
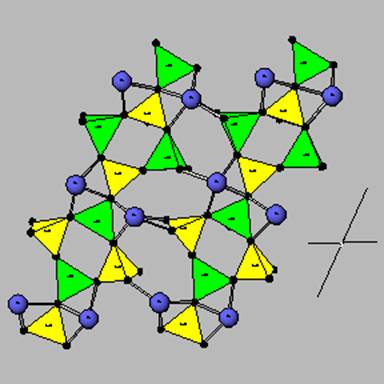 This is anorthite, the Ca plagioclase.
This is anorthite, the Ca plagioclase.
The Oxides
In both primary and secondary minerals, oxides are important (esp. in secondary
minerals). Below are a few examples of some important oxides. It will be
noted whether they are primary or secondary.
This is gibbsite
– discussed a bit above. Gibbsite is a secondary mineral (
Al(OH)3) that forms in rigorous weathering environments (where
Si is primarily lost).
 This is hematite
, one of the major Fe oxides in soils (Fe2O3). Hematite forms in warm and
dry environments and is especially expressed as soils age.
This is hematite
, one of the major Fe oxides in soils (Fe2O3). Hematite forms in warm and
dry environments and is especially expressed as soils age.
 This is the Fe oxide geothite
, a common secondary Fe oxide in wetter environments. Geothite has a more
reddish brown (ochre) color than hematite (red).
This is the Fe oxide geothite
, a common secondary Fe oxide in wetter environments. Geothite has a more
reddish brown (ochre) color than hematite (red).
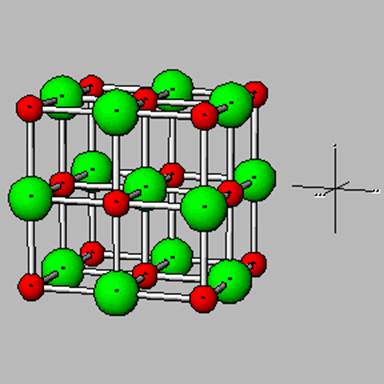
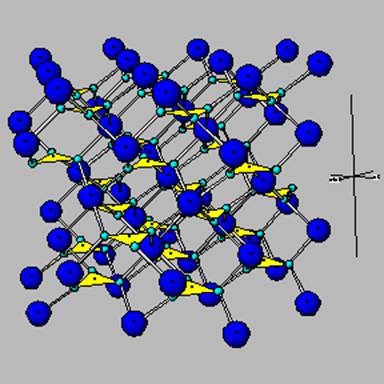
Carbonates
Ca released from the array of silicate minerals above normally travels all the way to the ocean in humid environements. In semiarid to arid conditions, it combines with carbonate in water to form calcite – the mineral shown below .

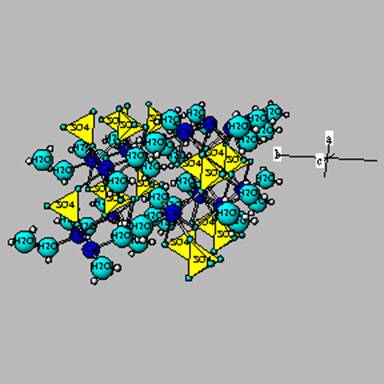
Sulfates
In arid environments, most elements are not removed from the soil.
If sulfate is present (from rocks, atmosphere, etc), it can combine with
Ca to form
gypsum – which is shown below.
Halides
In hyperarid enviroments, cations as soluble as Na and Cl combine to form common “salts”. Halite (NaCl), is shown below.