Physiologically Competent white adipose tissue on a chip
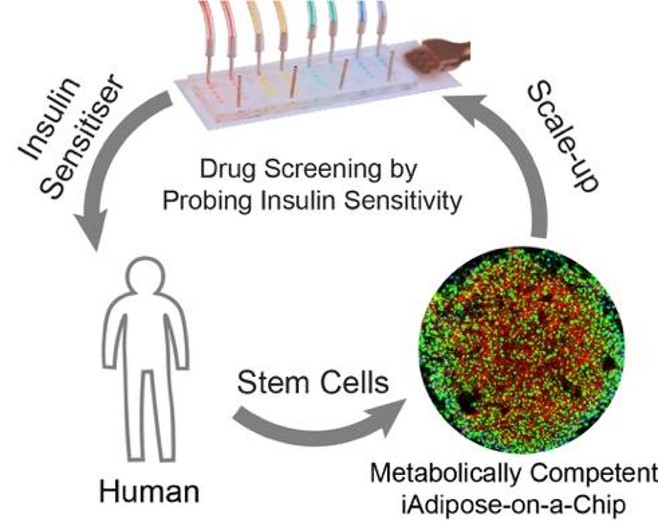
Obesity is a global pandemic, particularly severe in the US, where over 70% of adults are overweight including 40% with obesity [CDC: https://www.cdc.gov/nchs/fastats/obesity-overweight.htm]. White adipose tissue (WAT) is a critical organ in obesity and a primary target for treatment discovery, yet a reliable human in vitro model has been lacking due to difficulties in maintaining viability, maturity, and functionality. To address these challenges, we reconstructed a WAT within microphysiological system (WAT-MPS, or fat-chip) using human preadipocytes, mesenchymal stem cells, and induced pluripotent stem cells (hiPSCs). Our WAT-MPS is physiologically comparable to primary adipocytes from human biopsies and maintains over 90% viability for up to six months. With its robust responses to hormones in lipid and glucose metabolism, this fat-chip is an excellent platform for studying obesity and related diseases.
Multi-organ systems: fat-liver
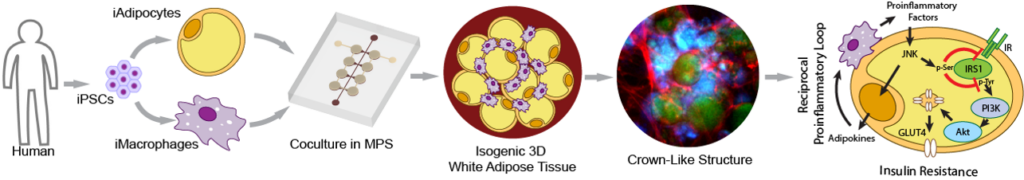
Interorgan crosstalk with WAT is a critical mechanism in triggering systemic metabolic disorders in obesity, but traditional in vitro cultures struggle to model this complexity. The organ-chip platform is particularly suited to mimicking interorgan crosstalk by circulating culture medium between connected chips. Therefore, it is plausible to connect our health and/or obese fat-chips to other organs to study obesity-induced diseases. The liver, being the most relevant metabolic organ due to its direct exposure to factors released from visceral fat through the portal vein, is a key focus. Here, we developed an interconnected fat-liver-chip consisting of isogenic hiPSC-derived white adipocytes, hepatocytes, and macrophages. We further utilized our MPS to identify and characterize pharmacological intervention strategies for hepatic steatosis and systemic insulin resistance. These efforts establish our human isogenic fat-liver MPS as an effective alternative to animal models for studying the etiology and therapy of highly prevalent metabolic diseases. Furthermore, we are working to interconnect more organs in-line, such as fat-liver-islet to form a minimal set of obesity-induced type-2 diabetes milieu, or fat-heart to study obesity-associated cardiac diseases. Our ultimate goal is to emulate a human-on-chip model for studying obesity-associated diseases across multiple organs and tissues.
Engineering and Interrogation of Functional Sexually Dimorphic Liver-Fat in a Microphysiological System
Although both men and women are both susceptible to obesity and these associated diseases (T2D and Fatty Liver), the risk associated varies per sex. Sex differences in the endocrine and immune systems contribute to these observations, recent studies suggest that sex-specific genetic architecture also influences human phenotypes, including reproductive, physiological and disease traits. It is likely that an underlying mechanism is differential gene regulation in males and females, particularly in sex steroid-responsive genes. We are working to establish an isogenic microphysiological model that can recapitulate these effects and demonstrate sex-specific differences.