Can the Garbelotto lab analyze wood chips for pathogens?
The lab can, upon request, analyze wood chips at cost ($90 US dollars per sample). Testing would confirm the presence or absence of any known dangerous pathogens.
matteolab.org
The lab can, upon request, analyze wood chips at cost ($90 US dollars per sample). Testing would confirm the presence or absence of any known dangerous pathogens.

 SOD is an exotic disease caused by the microscopic pathogen Phytophthora ramorum, estimated to have been introduced into California 20-25 years ago from unknown region of the world.
SOD is an exotic disease caused by the microscopic pathogen Phytophthora ramorum, estimated to have been introduced into California 20-25 years ago from unknown region of the world. and a foliar infection. Tanoaks are the only tree species that can spread the the disease and die from it as well. When P. ramorum infects oaks and tanoaks it destroys the cambium under the bark and effectively girdles the tree. Girdled trees are doomed, but can survive for 1 to 5+ years thanks to stored resources and their natural tolerance to drought.
and a foliar infection. Tanoaks are the only tree species that can spread the the disease and die from it as well. When P. ramorum infects oaks and tanoaks it destroys the cambium under the bark and effectively girdles the tree. Girdled trees are doomed, but can survive for 1 to 5+ years thanks to stored resources and their natural tolerance to drought.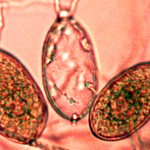 percipitation.
percipitation.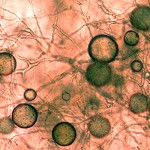



What’s in a name? Two of the most serious wood pathogens of conifers worldwide have been renamed by U.C. Berkeley and US Forest Service scientists. Because of the significant economic and scientific importance of these organisms, and because one of them was introduced in Europe by US soldiers during World War II, this is a long-awaited publication.


Fungi are a critical component of the diversity and function of terrestrial ecosystems. Pathogens and mycorrhizal fungi receive a large, direct share of net primary productivity, and wood decay and mycorrhizal fungi play a critical role in the cycling of key plant macronutrients. However, the biodiversity and community dynamics of these organisms are still poorly resolved, as is the extent to which they control plant and animal community structure. For my thesis work I used molecular, GIS, and isotopic techniques to examine a number of topics related to the community assembly and symbiotic dynamics of ectomycorrhizal fungi.
The first part of my thesis applied the theory of island biogeography to ectomycorrhizal “tree-islands” patches of host trees embedded in a non-host matrix as a way to look for evidence that immigration and extinction affect ectomycorrhizal assemblages. These tree-islands generally conformed to the expectations of island biogeography theory larger tree islands housed more species of ectomycorrhizal fungi, and more isolated tree islands had fewer species of ectomycorrhizal fungi. This work led to one of the few published species-area relationships for fungi and provided good evidence for a competition-colonization tradeoff in ectomycorrhizal communities. The second portion of my thesis work used manipulative experiments to assess the effects of fire on ectomycorrhizal assemblage structure and host plant relations. We found that simulated fire simplified and shifted the ectomycorrhizal assemblage colonizing seedlings. We also found that the species with the greatest increase in abundance from simulated fire also had the most heat tolerant spores, indicating that this may be an important mechanism for changes in post-fire assemblage structure. We also found evidence that the ectomycorrhizal plant-fungal symbiosis remained mutualistic despite dramatic changes in the soil environment after simulated fire.
From this work we have found evidence for the importance of both stochastic ecological processes, such as immigration, as well as deterministic ecological processes, such as niche partitioning. Because most assemblages are likely affected by both sets of processes, I believe a key challenge in moving fungal community ecology forward is to synthesize results from both types of studies and determine the spatial and temporal scales at which they are most important in determining species’ abundance and distribution.

The U.C. Berkeley Forest Pathology Lab directed by Matteo Garbelotto and the Gonthier Lab at the University of Turin, Italy, have jointly discovered the US military transported H. irregulare from North America to Italy in 1944, and have spearheaded most of the research on its invasion process. In October 2013, thanks to their efforts, Heterobasidion irregulare was included in the alert list of fungi by the European and Mediterranean Plant Protection Organization (EPPO). The H. annosum species complex comprises necrotrophic pathogens regarded as the most destructive disease agents of conifers. In the past 40 years, H. annosum s.l. has been the object of more than 1,700 scientific papers, which makes it one of the most intensively studied forest fungi. The complete genome sequence of the fungus is now available, making H. annosum s.l. the first sequenced plant pathogenic homobasidiomycete. Furthermore, it is one of the few examples of a forest pathogen that can be and has been controlled in managed forests. The understanding that two sister allopatric species are now sympatric because of a human-linked intercontinental movement of one of the two, provides the scientific community with the opportunity to understand in-depth the ecological similarities and differences among species within the complex.Pertinent publications from the Berkeley-Turin groups: Giordano, L., Gonthier, P., Lione, G., Capretti, P., M., Garbelotto, M. (2013) The saprobic and fruiting abilities of the exotic forest pathogen Heterobasidion irregulare may explain its invasiveness. Biol Invasions DOI 10.1007/s10530-013-0538-4 PDFGarbelotto M., Gonthier, P. (2013) Biology, Epidemiology, and Control of Heterobasidion Species Worldwide. Annu. Rev. Phytopathol. 51:39-59. PDFGarbelotto M., Guglielmo, F., Mascheretti, S., Croucher, P., Gonthier, P. (2013) Population genetic analyses provide insights on the introduction pathway and spread patterns of the North American forest pathogen Heterobasidion irregulare in Italy. Molecular Ecology, 22: 4855-4869 doi: 10.1111/mec.12452. PDF Gonthier, P., Lione, G., Giordano, L., Garbelotto, M. (2012) The American forest pathogen Heterobasidion irregulare colonizes unexpected habitats after its introduction in Italy. Ecological Applications 22(8) 2135-2143. PDF Gonthier, P. & Garbelotto, M. (2011) Amplified fragment length polymorphism and sequence analyses reveal massive gene introgression from the European fungal pathogen Heterobasidion annosum into its introduced congener H. irregulare. Molecular Ecology 20, 2756-2770 PDF Otrosina, W. & Garbelotto, M. (2009) Heterobasidion occidentale sp. nov. and Heterobasidion irregulare nom. nov.: A disposition of North American Heterobasidion biological species. Mycological Research, doi:101016/j mycres 2009.09.001 PDF Garbelotto, M., Linzer, R., Nicolotti, G., & Gonthier, P. (2009) Comparing the influences of ecological and evolutionary factors on the successful invasion of a fungal forest pathogen. Biol Invasions, doi: 10.1007/s10530-009-9514-4 PDF Linzer, R. E., Otrosina, W. J., Gonthier, P., Bruhn, J., Laflamme, G., Bussieres, G., & Garbelotto, M. (2008) Inferences on the phylogeography of the fungal pathogen Heterobasidion annosum, including evidence of interspecific horizontal genetig transfer and of human-mediated, long-range dispersal. Mol. Phylogenet. Evol., 46, 844-862. PDF Gonthier, P., Nicolotti, G., Linzer, R., Guglielmo, F., & Garbelotto, M. (2007) Invasion of European pine stands by a North American forest pathogen and its hybridization with a native infertile taxon. Molecular Ecology, 16, 1389-1400. PDF |
|